Phenol: Difference between revisions
Jump to navigation
Jump to search
JeffEvarts (talk | contribs) |
|||
| (2 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
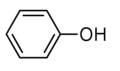
{{Compound|name=Phenol|chemf=C6H5OH | {{Compound|name=Phenol|chemf=C6H5OH|mm=94.11|density=1.07|mp=40.5|bp=181.7|ior=1.5425|stp_p=crystalline solid|stp_q=white|nfpa_h=3|nfpa_f=2|nfpa_o=COR|sol_aq=83}} | ||
==Uses== | ==Uses== | ||
{{Justify}} | {{Justify}} | ||
| Line 28: | Line 28: | ||
*: {{#Chem: C6H6 + H2SO4 = C6H5SO3H + H2O}} | *: {{#Chem: C6H6 + H2SO4 = C6H5SO3H + H2O}} | ||
*: {{#Chem: C6H5SO3H + 2 NaOH → C6H5OH + Na2SO3 + H2O }} | *: {{#Chem: C6H5SO3H + 2 NaOH → C6H5OH + Na2SO3 + H2O }} | ||
* From [[benzene]] via Fenton's reagent: | |||
*: {{#Chem:c6h6 + H2O2 + FeSO4 = C7H5OH}} | |||
* From [[benzene]] and nitrous oxide | * From [[benzene]] and nitrous oxide | ||
*: {{#Chem: C6H6 + N2O = C6H5OH + NO}} | *: {{#Chem: C6H6 + N2O = C6H5OH + NO}} | ||
Latest revision as of 18:14, 21 July 2021
Uses
Justification Questioned
Other
- Cleaning
- Antiseptic
- Naturally occurring, easily obtainable aromatic compound for organic syntheses, e.g. 2,6-xylenol.
Natural occurrence
- Phenol occurs naturally in tars and crude oil
Hazards
- Toxic
- Flammable
- Corrosive
Production
Extraction
Coal Tar
- distill coal tar and retain what distills between 170° and 230°C
- redistill and retain what distills between 180°C and 183°C
Synthesis
- From salicylic acid via thermal decomposition
- C7H6O3(s){C6H5OH(l) + CO2(g)145-165°C}→
- C7H6O3(s)
- From benzene via sulfuric acid and sodium hydroxide
- C6H6 + H2SO4 → C6H5SO3H + H2O
- C6H5SO3H + 2 NaOH → C6H5OH + Na2SO3 + H2O
- From benzene via Fenton's reagent:
- c6 h6 + H2O2 + FeSO4 → C7H5OH
- From benzene and nitrous oxide
- C6H6 + N2O → C6H5OH + NO
- From oxidation of toluene (DOW Chemical process)
- C6H5CH3 + 2 O2 → C6H5OH + CO2 + H2O