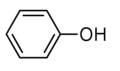
Phenol
Jump to navigation
Jump to search
Uses
Justification Questioned
Other
- Cleaning
- Antiseptic
- Naturally occurring, easily obtainable aromatic compound for organic syntheses, e.g. 2,6-xylenol.
Natural occurrence
- Phenol occurs naturally in tars and crude oil
Hazards
- Toxic
- Flammable
- Corrosive
Production
Extraction
Coal Tar
- distill coal tar and retain what distills between 170° and 230°C
- redistill and retain what distills between 180°C and 183°C
Synthesis
- From salicylic acid via thermal decomposition
- C7H6O3(s){C6H5OH(l) + CO2(g)145-165°C}→
- C7H6O3(s)
- From benzene via sulfuric acid and sodium hydroxide
- C6H6 + H2SO4 → C6H5SO3H + H2O
- C6H5SO3H + 2 NaOH → C6H5OH + Na2SO3 + H2O
- From benzene and nitrous oxide
- C6H6 + N2O → C6H5OH + NO
- From oxidation of toluene (DOW Chemical process)
- C6H5CH3 + 2 O2 → C6H5OH + CO2 + H2O