Water
| Chemical formula | H2O |
|---|---|
| OTP appearance | clear liquid |
| Index of refraction | 1.333 |
| Molar Mass(g/mol) | 18 |
| Enthalpy of Formation(kJ/mol) | −187.80 |
| Density(g/cc) | 1 |
| Melting Point(°C) | 0.1 |
| Boiling Point(°C) | 100 |
| Solubility in ethanol(g/L) | misc |
| NFPA 704 |
Water, in the English language, is an inexact term. This article deals with the chemical compound H2O: pure water.
Uses
Primary
- Drinking water is essential to human life
- In chemistry, it is a common polar solvent
- Ice is a key thermal absorption material
Secondary
- Expansion on freezing is a key mechanic in early stone splitting
Natural occurrence
- Water occurs naturally
- as fresh water (ultimately derived from rain)
- Rain
- Springs
- Rivers
- Lakes
- and salt water from
- oceans
- seas
- salt lakes
- brine deposits
- as fresh water (ultimately derived from rain)
- Structural water occurs in many minerals
- Many plants contain an abundance of water
Hazards
- Drowning
- Overconsumption (>= 2L/hr for multiple hours when normally hydrated) can lead to hyponatremia
Production
Extraction
- Pure water can be extracted from fresh water and salt water by distillation.
Synthesis
from hydrogen gas
- Burn hydrogen in air, directing the combustion products to a condenser
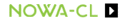
- 2 H2 + O2{H2O(v)combustion}→
- 2 H2 + O2
- Condense the water formed
As a side product
Many chemical reactions, particularly acid/base reactions (e.g. acetic acid and calcium hydroxide), produce water as a side product.
- 2 CHCOOH + Ca(OH)2 → Ca(CH3COO)2 + 2 H2O // acetic acid and calcium hydroxide produce calcium acetate and water
Testing
Quantitative
- Density
- Boiling Point
- Melting point,
Qualitative
- Visual: Clear
- Smell: None
- Taste: Charctristic
- Approximate Viscocity : Characteristic
Purification
Filtration
- Passing water through clean, fine sand will remove some microorganisms.
- Passing water through charcoal or activated charcoal will remove toxins such as cyanide.
Arranging a filter with layers of sand and charcoal is an effective way to produce relatively clean (unpotable) water. It should still be boiled before drinking.
Distillation
- Distilled water is water that has been condensed from a gas and stored in a clean container. Distilled water has no dissolved solids and is completely sterile.
- distil water
- discard residue
- distillate is distilled water
Fractional distillation
Other liquid adulterants which form azeotropes with water (ammonia, ethanol, sulfuric acid, etc) can be removed by fractional distillation, leaving a pure water fraction and a contaminant + water fraction. Or alternatively specific contaminants can be salted out: add calcium hydroxide to sulfuric-acid contaminated water to produce insoluble calcium sulfate and more water:
- 2 CaOH + H2SO4(aq) → Ca2SO4(s) + 2 H2O
Other
- The mineral witherite is sometimes used for the removal of calcium and magnesium sulfates from water via precipitation
- 2 BaCO3(s) + CaSO4(aq) + MgSO4(aq) → CaCO3(s) + MgCO3(s) + BaSO4(s)
Storage
Store in watertight containers.
Disposal
Water can be safely discarded into the environment
