Urea: Difference between revisions
Jump to navigation
Jump to search
JeffEvarts (talk | contribs) |
|||
| (4 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
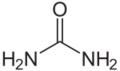
{{Compound|aka=diamino carbonyl|chemf=OC(NH2)2|sol_aq=≥1000|sol_et=50|mp=135|mm=60|density=1.32|pharm=yes|listed_who=yes|decomp={{DecompRow|temp=unk|input=urea|output={{#Chem: NH3, CO2}}}}|industrial=yes|nowa=yes}} | |||
{{Compound|aka=diamino carbonyl|chemf=OC(NH2)2|sol_aq=≥1000|sol_et=50|mp=135|mm=60|density=1.32| | |||
__TOC__ | __TOC__ | ||
A major source of organic [[nitrogen]]. | A major source of organic [[nitrogen]]. | ||
| Line 30: | Line 29: | ||
The crystals are urea | The crystals are urea | ||
=====further separation===== | =====further separation===== | ||
<div style='margin-left:1em;'> | |||
;Reactants | ;Reactants | ||
* [[Urine]] | * [[Urine]] | ||
| Line 43: | Line 43: | ||
* Gas bubbling equipment | * Gas bubbling equipment | ||
* (Optional) Distillation equipment | * (Optional) Distillation equipment | ||
; Process | |||
# Boil 10L of [[urine]] down to 1L (containing ~93g, 6.6M of [[urea]]) | # Boil 10L of [[urine]] down to 1L (containing ~93g, 6.6M of [[urea]]) | ||
# Filter | # Filter | ||
| Line 58: | Line 58: | ||
#: '''NB:''' It may be worth condensing the ethanol vapors | #: '''NB:''' It may be worth condensing the ethanol vapors | ||
# The residue is urea. It should crystallize | # The residue is urea. It should crystallize | ||
</div> | |||
=====via oxalic acid===== | =====via oxalic acid===== | ||
* Precipitate from [[urine]] using [[oxalic acid]], then extract again using [[calcium hydroxide]]. Detoxify the calcium oxalate by thermal decomposition. | * Precipitate from [[urine]] using [[oxalic acid]], then extract again using [[calcium hydroxide]]. Detoxify the calcium oxalate by thermal decomposition. | ||
| Line 78: | Line 80: | ||
# Until no further residue is collected and the crystals are as pure as desired | # Until no further residue is collected and the crystals are as pure as desired | ||
===Synthesis=== | ===Synthesis=== | ||
====industial==== | |||
* Combine [[carbon dioxide]] with [[ammonia]] <ref>{{Cite patent|4061675A}}</ref> | |||
*: {{#Chem: CO2 + 2NH3 = CO(NH2)2 + H2O}} | |||
====Wohler==== | ====Wohler==== | ||
* Combine '''silver cyanate''' with [[ammonium chloride]]. | * Combine '''silver cyanate''' with [[ammonium chloride]]. | ||
Latest revision as of 20:32, 20 October 2023
A major source of organic nitrogen.
Uses
Primary
- Industrial chemical
- Fertilizer (46-0-0)
Secondary
- Feedstock (through natural decomposition) to ammonia
- Feedstock for urea nitrate for cold packs
- Pharm: (ointment) Skin treatment
Natural occurrence
Hazards
Production
Extraction
from urine
Approximately 9.3g are expected in a liter of fresh human urine. Delay in processing allows bacteria to convert the urea to ammonia.
simple evaporation
- Dry urine to solids
- Repeat
- Dissolve in ethanol
- filter
- discard residue
- evaporate alcohol
- gather crystals
- Until nothing but clear crystals remains
The crystals are urea
further separation
- Reactants
- Products
- Urea
- Mineral salts rich in Sodium chloride (roughly sea salt)
- Organic sludge rich in phosphate and proteins
- Equipment
- Liquid Dryer/Evaporater
- Gas bubbling equipment
- (Optional) Distillation equipment
- Process
- Boil 10L of urine down to 1L (containing ~93g, 6.6M of urea)
- Filter
- Residue contains organics, calcium and other phosphates, can be retained to produce phosphorus or burned and disposed
- Filtrate contains sodium chloride and urea
- Evaporate water by gentle heating (≤ 133°C)
- Residue is mostly sodium chloride and urea
- Wash residue with hot concentrated ethanol several times, combine washings
- NB: Urea dissolves easily, salt does not.
- IF (two layers form in the washings)
- Separate the aqueous layer and evaporate separately, producing mineral salts
- ENDIF
- Evaporate ethanol washings
- NB: It may be worth condensing the ethanol vapors
- The residue is urea. It should crystallize
via oxalic acid
- Precipitate from urine using oxalic acid, then extract again using calcium hydroxide. Detoxify the calcium oxalate by thermal decomposition.
- CO(NH2)2 + H2(COO)2 → C3H6N2O5
- C3H6N2O5 + Ca(OH)2 → CO(NH2)2 + Ca(COO)2 + 2 H2O
- Ca(COO)2{CaCO3 + CO≥600°C}→
via nitric acid
- Combine urine and nitric acid, producing urea nitrate crystals
- Filter
- Discard filtrate
- Repeat
- Until no further residue is collected and the crystals are as pure as desired
Synthesis
industial
- Combine carbon dioxide with ammonia [1]
- CO2 + 2 NH3 → CO(NH2)2 + H2O
Wohler
- Combine silver cyanate with ammonium chloride.
- AgNCO + NH4Cl → CO(NH2)2 + AgCl
See Also
References
- ↑ US patent 4061675A
Link courtesy Google